Since 2006, the Hans and Ilse Breuer Foundation has awarded the Alzheimer Research Prize, endowed in principle with 100,000 euros, to a total of 25 researchers – individually or divided into two. The prize is awarded to scientists who perform outstandingly well in the field of Alzheimer’s research or similar dementias.
Until 2017, scientists were able to apply for the Alzheimer’s Research Award, since 2018 the Foundation’s Scientific Advisory Board is looking for excellence and nominates suitable candidates. The Scientific Advisory Board currently includes Dr. Daniel Fleck, Prof. Christian Haass, Prof. Dr. Manuela Neumann, Prof. Dr. Dr. Pierluigi Nicotera, Prof. Dr. med. Steffi G. Riedel-Heller and Dr. Stacie Weninger.
You are welcome to propose interesting projects or research results at home and abroad in the field of Alzheimer’s research, dementia research and the research of similar aging diseases to us.

Award winners
Find out more about the winners of our Alzheimer’s Research Award and their research projects.

The growing number of people living with dementia is a major public health challenge. To change this prospect, research is currently running along two complementary lines: one focusing on developing curative pharmacological treatments, and the other on developing preventive measures targeting modifiable risk and protective factors. While several risk and protective factors for dementia have been identified in recent years, little is still known about their true causal effect, differences among individuals in their susceptibility to these risks, the way factors relate to each other, and their underlying brain mechanisms. This hampers the development and implementation of effective interventions and preventive strategies. In addition, the general population is still largely unaware of the relationship between lifestyle and dementia risk and means to raise awareness are mostly lacking, still.
With my research group, three major themes exist: The first is the study of risk and protective factors for cognitive decline and dementia in the general and clinical populations. A focus of this theme is a better understanding of the individual as well as shared (environmental) risk factors as well as underlying neural mechanisms that shape brain health during the life-course. Differences between individuals by sociodemographic and genetic background is taken into account. Another main theme is the translation of epidemiological findings into universal, selective and indicated prevention programs by developing tools and lifestyle programs that help people to adopt a brain-healthy lifestyle. Third, we inform the public about our research findings through lectures, public outreach activities and health campaigns to raise awareness for the relationship between lifestyle and dementia risk.
For this, we conduct observational, experimental and implementation studies, and collaborate within national and international consortia, and with private and societal partners like the Alzheimer Nederland, the Dutch Brain Foundation, municipal health services, the Ministry of Health and the World Health Organization.
The research award allows me to strengthen our understanding of individual differences in risk and protective factors by studying their associations with underlying brain mechanisms such as total and regional brain volume loss and vascular brain damage. A special focus will be on the brain’s structural and functional connectivity, that is, the way (groups of) neurons are connected to each other in the brain and how a healthy lifestyle might improve this connectivity. This is important since research has shown that people with better connected brains have a lower risk for dementia. Another focus will be on how risk and protective factors, as well as underlying brain mechanisms, differ between individuals with first cognitive symptoms compared to cognitively-healthy individuals. This will inform us about the need for developing specialized interventions for people at high risk for dementia.
Resume
Sebastian Köhler is Professor of Neuroepidemiology within the Department of Psychiatry and Neuropsychology and principal investigator at the Alzheimer Centrum Limburg, both at Maastricht University. He was trained in neuropsychology at Radboud University Nijmegen and in epidemiology at the London School of Hygiene and Tropical Medicine, and received a Ph.D. in neuropsychiatry from Maastricht.
Sebastian Köhler leads the research line Risk and Prevention of the Alzheimer Centrum Limburg. which specializes in population research into risk and protective factors for cognitive decline and dementia. The mission of the research line is to help people grow old with good mental and brain health through the translation of epidemiological findings into preventive interventions at both the individual and the population level. His group developed the internationally known LIBRA score for dementia prevention, the app ‚MijnBreincoach‘ to stimulate a brain-healthy lifestyle, the public health campaign ‚We are the medicine ourselves‘ and the risk online portal breinzorg.nl for risk self-management for patients of the memory clinic.
Sebastian Köhler is deputy head of the division ‚Cognitive Neuropsychiatry and Clinical Neuroscience‘ of the Mental Health and Neuroscience Research Institute and on the management board of the Alzheimer Centrum at Maastricht University. He is an expert consultant for the Brain Health Unit of the World Health Organization and Alzheoimer Europe and leads the INTERDEM Task Force Prevention. He (co-)leads the community-based Maastricht Ageing Study and The Maastricht Study and several intervention studies into dementia risk reduction including the Netherlands Dementia Prevention Initiative (NDPI), the work package ‚ Prevention‘ within the national ABOARD consortium, the FINGER-NL multi-centre trial into lifestyle and cognition in old age, and the new LIGHT study, a multi-centre trial into lifestyle coaching at the memory clinic.
Sebastian Köhler was born in Schleswig, Germany, and grew up in Viersen before moving to Nijmegen in the Netherlands for his studies of psychology. Since the start of his Ph.D. in Maastricht in 2005, he lives in Aachen, happily with his wive and four sons.

Neurodegenerative diseases are characterized by the abnormal accumulation of a limited number of proteins in the central nervous system. These proteins include tau, amyloid beta, alpha-synuclein, and TDP-43. Mutations in the genes encoding each of these proteins lead to assembly and inheritance of the disease, suggesting a causal role. The assembly begins at disease-specific sites in the brain and then spreads over years to contiguous regions in the CNS, leading to characteristic patterns of neurodegeneration and clinical manifestations. The assembly is thought to contribute to neurodegeneration by acquiring a toxic function and possibly losing function under certain circumstances. However, the mechanisms of assembly formation, progression, and toxicity are unclear. A molecular understanding of these mechanisms could lead to successful strategies for early diagnosis and effective treatment of neurodegenerative diseases that do not currently exist.
With support from the Hans and Ilse Breuer Foundation Alzheimer’s Research Award, Benjamin Ryskeldi-Falcon aims to study the molecular structures of assembled TDP-43 from donated brains of patients with neurodegenerative diseases using cryo-electron microscopy (cryo-EM). Assembly of TDP-43 underlies nearly all cases of the motor neuron disease amyotrophic lateral sclerosis (ALS) and about half of cases of frontotemporal dementia (FTLD), the third most common neurodegenerative disease after Alzheimer’s and Parkinson’s disease. In addition, TDP-43 assembly appears to be responsible for up to a quarter of cases of late-stage Alzheimer’s disease, a condition called limbic-predominant age-related TDP-43 encephalopathy (LATE). In about half of Alzheimer’s cases, TDP-43 assembly occurs along with tau and amyloid beta assembly, leading to more severe disease courses. Recently, Benjamin Ryskeldi-Falcon determined the structures of assembled TDP-43 from patients with ALS and FTLD, revealing that TDP-43 forms a kind of thread-like structure called amyloid. However, the structures of assembled TDP-43 in other neurodegenerative diseases are not known. These structures will guide research into the molecular mechanisms of TDP-43 assembly and directly inform the development of diagnostic and therapeutic agents that target assembled TDP-43.
Resume
Benjamin Ryskeldi-Falcon was born in Edinburgh, United Kingdom, and graduated from University College London with a Bachelor of Science in Human Genetics. He completed his studies with Michel Goedert at the MRC Laboratory of Molecular Biology (LMB) and received his PhD in Molecular Biology from the University of Cambridge in 2016. From 2016 to 2019, he worked as a postdoctoral researcher with Michel Goedert and Sjors Scheres at LMB, where he contributed to the determination of cryo-electron microscopy (cryo-EM) structures of assembled tau in neurodegenerative diseases such as Alzheimer’s disease, frontotemporal dementia (FTD) and chronic traumatic encephalopathy (CTE). Since October 2019, Benjamin has been leading a research group at LMB that focuses on the molecular mechanisms of protein assembly in neurodegenerative diseases. He was awarded the Alzheimer’s Research UK Rising Star Award in 2019 for his research and was elected to the European Molecular Biology Organisation (EMBO) Young Investigator Programme in 2022.

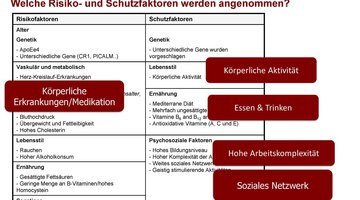
The potential for risk reduction and prevention of dementia is considered to be very high. To this end, particular attention is being paid to modifiable risk factors that have been shown to be associated with an increased likelihood of dementia in old age. Currently, the following factors are recognized: low education in early life; hearing loss, traumatic brain injury, hypertension, obesity, excessive alcohol consumption in midlife; diabetes mellitus, depression, physical inactivity, smoking, social isolation, and exposure to air pollution in later life. If these 12 risk factors were eliminated, at least 40% of all dementias could be prevented. Most of the evidence on modifiable risk factors comes from epidemiological research based on long-term observational studies. International studies are continually seeking to identify additional risk factors. Increasingly, intervention studies are investigating whether targeted modification of such factors can reduce cognitive decline and dementia risk. Typically, the so-called multicomponent studies test the efficacy of complex lifestyle interventions (simultaneous programs to promote physical, cognitive, and social activity; nutritional counseling; mental state; management of blood pressure and diabetes) in individuals at increased risk for dementia and/or in preclinical or prodromal stages of dementia. Results to date have been mixed, suggesting small effects, if any, on cognitive performance in specific groups of individuals. This has several methodological reasons, but most importantly raises the question of how best to approach dementia prevention to maximize potential. This is the focus of Susanne Röhr’s current research, which receives substantial support from the Alzheimer Research Award of the Hans and Ilse Breuer Foundation. The central assumption here is that, in addition to opportunities for behavioral prevention, i.e., the promotion of a healthy lifestyle, greater emphasis must be placed on opportunities through relationship prevention. Initial studies by Susanne Röhr with data from the Leipzig LIFE adult study point to the importance of social determinants for brain health. In particular, structural determinants (e.g., education, work and income, access to health care, health insurance, living spaces and environments) are closely related to inequalities in health and contribute to who develops dementia syndrome and at what age. Therefore, research and development of health policy strategies that prioritize relationship prevention and aim to create equitable and sustainable living conditions that enable access and choices for healthy lifestyles is needed – that, unlike outlined multicomponent programs, would benefit not only individuals but society across generations.
Resume
Susanne Röhr was born in Rochlitz in 1981. She first studied journalism at the University of Leipzig, completed a traineeship at the Rheinische Post and worked for the Freie Presse and MDR, among others. She then obtained a bachelor’s degree (University of Leipzig) and master’s degree (Chemnitz University of Technology) in psychology, and in 2017 completed her doctorate at the Institute of Social Medicine, Occupational Medicine and Public Health (ISAP), University of Leipzig, on early cognitive symptoms in preclinical dementia. From 2018 to 2020, Susanne Röhr led the research group „Epidemiology & Population Brain Health“ at ISAP, which conducted age cohort studies (e.g. AgeCoDe / AgeQualiDe) and intervention studies (e.g. AgeWell.de, Sanadak), among others. Other stops included a postdoctoral residency at UNSW Sydney, Australia, 2018, and Trinity College Dublin, Ireland, 2020-2021. Susanne Röhr is a lifetime Atlantic Fellow for Brain Health Equity at the Global Brain Health Institute (Trinity College Dublin / UC San Francisco) and Atlantic Institute (Oxford). She completed her habilitation in the field of Epidemiology and Public Health at the Medical Faculty of the University of Leipzig in 2021 on approaches to dementia prevention. In 2022, Susanne Röhr accepted an appointment to a research professorship at Massey University in New Zealand, where she will expand population-based studies on cognition and age health.

A known pathological feature of Alzheimer’s disease is neurofibrillary tangles containing the protein tau. Tau aggregates first form in a few neurons, from where they spread to other brain regions. We hypothesize that the process of tau oligomerization ultimately leads to neuronal dysfunction and the typical symptoms of Alzheimer’s dementia. Another protein that is deposited in the brain in up to 50% of Alzheimer’s patients, thereby disrupting its function, is the TDP-43 protein. This protein is known to play an important pathological role in other neurodegenerative diseases, such as frontotemporal dementia and amyotrophic lateral sclerosis. Alzheimer’s patients with both tau and TDP-43 aggregates show more severe brain shrinkage and cognitive decline than patients with only tau deposits, suggesting an important contribution of TDP-43 to neurodegeneration in Alzheimer’s disease. However, how TDP-43 deposits form in Alzheimer’s disease and whether TDP-43 and tau influence each other’s pathology is currently unknown. Financially supported by the 2021 Alzheimer’s Research Award, we would like to use test tube (in vitro) and cell experiments to investigate whether tau and TDP-43 reciprocally influence their aggregation and spread from cell to cell. Likewise, we would like to investigate which of the two pathologies occurs first in Alzheimer’s patients and how TDP-43 aggregates are taken up by neurons. This research will help us better understand the molecular basis of TDP-43 and tau dysfunction in Alzheimer’s disease and provide new mechanistic insights into AD and related dementias. An understanding of the molecular mechanisms of disease is an important prerequisite for the development of new approaches to the prevention, diagnosis and treatment of these previously incurable dementias.
Resume
Dorothee Dormann was born in Schorndorf and studied biochemistry at Eberhard Karls University in Tübingen. She subsequently completed her doctorate at the Rockefeller University in New York, where she received her Ph.D. in the field of cell biology/immunology in 2007. From 2007 – 2013, as a postdoctoral researcher in the laboratory of Christian Haass (Adolf-Butenandt-Institute, LMU Munich), she investigated the molecular causes of TDP-43 and FUS dysfunction in amyotrophic lateral sclerosis and frontotemporal dementia. From 2014 – 2021, she led an independent Emmy Noether junior research group at the Biomedical Center of LMU Munich. Since April 2021, Dorothee Dormann is Heisenberg Professor of Molecular Cell Biology at Johannes Gutenberg University Mainz and Adjunct Director at the Institute of Molecular Biology (IMB). Her research focuses on the molecular mechanisms of protein mislocalization and aggregation in neurodegenerative diseases. She has already been awarded the Heinz Maier-Leibnitz Prize of the DFG (2014) and the Paul Ehrlich and Ludwig Darmstädter Young Investigator Award (2019) for her research. Dorothee Dormann is married and has two children.
Henne Holstege, Assistant Professor at the Amsterdam UMC.
Why do some people suffer from dementia symptoms at the age of 70, while others live to be over 100 with no signs of cognitive decline? An extreme example is Mrs. van Andel-Schipper: She reached 115 years without a trace of dementia. Her mother also lived to be 100, and her mind was also sharp until the day she died. In fact, previous research has shown that reaching extreme old age with maintained cognitive health is found in some families. I found this so fascinating that I set out to investigate the genetic factors that play a role in escaping dementia. Mrs. Van Andel was just one person. To figure out how it is possible to reach extreme old age without dementia, more people like her who are extremely old and cognitively healthy need to be studied. For this reason, in 2013, I established the 100-plus cohort of cognitively healthy centenarians. As a result, we have now collected data from more than 400 healthy centenarians. First, we need to determine how well the brains of the over 100 are functioning. To do this, we visit the centenarians and test their brain function with neuropsychological tests. We test whether the centenarians can still plan, reason or calculate, and whether their memory functions still work properly. We also examine the participants‘ blood. About one-third of those over 100 are willing to donate their brains after they die. From the blood, we isolate DNA and also the cells of the immune system. More and more studies are showing that the immune system plays a role in the development of dementia. Indeed, our results show that the genetic risk factors associated with the onset of dementia are often involved in immune system function. Part of our studies involves using genetic material and the blood cells of centenarians to better understand how their immune systems differ from people who have dementia. Our genes determine whether we grow tall or short, whether someone has blond or black hair, brown or green eyes. But the susceptibility to developing Alzheimer’s disease is also about 60-80%, defined by our genes. We can use this to our advantage: We can use genetics to predict who will eventually develop dementia before the disease manifests. This allows us to apply individualized treatment before the disease has done any damage to the brain. If we can figure out how the genes of centenarians are involved in maintaining their brain health, we may be able to mimic the function of those genes by developing a drug for dementia that accomplishes the same thing. In this way, we can learn from the centenarians how to maintain human brain health. In the centenarian genomes, we look at the genetic „abnormalities“ that prevent someone from developing dementia. In our study, we examine the extremes. In contrast, several inherited abnormalities are known to cause or increase dementia risk. For example, some genetic disorders cause a buildup of amyloid beta protein in the brain, and carriers of such a genetic disorder are almost certain to develop Alzheimer’s disease. Identifying these high-risk genes is also part of our study. We are now conducting an international trial that will examine more than 50,000 genomes of individuals with or without Alzheimer’s disease. Recently, we have found that certain abnormalities within new genes may also be involved in the development of dementia. While my own expertise is directly based on the genetic background of cognitive decline and its escape, much of our research depends on long-term collaborations with other researchers with other expertise both within and outside Amsterdam UMC, as well as with researchers abroad. I realize that it is only through these collaborative efforts that we can generate maximum revenue from the data and biomaterials we collect as part of the 100+ year study. It is a true privilege to work with so many inspired people with diverse expertise. Such collaborations, especially with human material, can only be possible if we ensure that international research agreements are adhered to. To continue these national and international collaborations, I would like to use the support of the 2020 Alzheimer’s Research Award from Hans and Ilse Breuer Foundation. I hope that our studies on the genetics of healthy centenarians, their immune systems and their brains can teach us how a human brain can remain healthy into old age and contribute to a future where far fewer people will suffer terrible symptoms due to dementia.
Resume
Henne Holstege was born in Rotterdam (The Netherlands) on November 14, 1975. She studied chemistry at Leiden University (The Netherlands) and graduated in 2001. In 2002, she obtained her PhD in the group of Jos Jonkers at the Netherlands Cancer Institute. Since 2010, she has been working at the Amsterdam University Medical Center in Clinical Genetics and at the Alzheimer Center. In 2013, she started the 100+ year study and has been an assistant professor at Amsterdam UMC since 2015. She currently leads the Clinical Genetics Unit, where the group studies the genomics of aging, with a focus on neurodegenerative diseases. Henne Holstege is married and has three daughters.
According to current figures, around 1.7 million people in Germany suffer from dementia. This makes it one of the most common diseases, and the trend is rising. Mainly older people are affected and the situations in which they live are very different. This is based on various life paths, experiences, concomitant diseases, regional and other factors.

The healthcare system is basically very well positioned to ensure good care for people with dementia. There are very good diagnostic methods, also in international comparison, a strong network of physicians in private practice, specialized facilities, various care services, etc. There are guidelines for care, and a wide range of counseling services is available from various providers. There are guidelines for care, and a wide range of counseling services is available from various providers. Nevertheless, there are great differences in care, and it is not yet possible to speak of systematic, high-quality care for all that is available and used throughout the country. This finding is based on my own research to date, but is also consensus in the general care landscape, as documented by the National Dementia Strategy adopted in mid-2020. In my research, the needs of people with dementia and their relatives relate to three major thematic blocks:
1. individualization of treatment and care of people suffering from dementia and their relatives. While dementia is a syndrome, treatment and care are individualized. People vary due to co-morbidities, some live alone, others do not, some are socially involved, understandings of health differ, goals in life, etc. The more individualized treatment and care is, the more effective it can be. This is my basic conviction and under this aspect I conduct scientific effectiveness and evaluation studies.
2. availability, access and quality of care. This refers to „the other“ side of care. In principle, everything is available, but do people use it? What are enabling and hindering conditions? For example, how does a service in a rural area have to be equipped to be used? How in a metropolitan area? There is no one model for all of Germany.
3. Relative burden and support. Most people are cared for at home. This is stressful and often relatives suffer physically and psycologically. How can the care system be changed to address this, how can this be improved?

A core concept in my research is stakeholder participation in research. I want to conduct research with effectiveness and impact on care. For this, it is important to work together with those affected. This concerns patients, relatives as well as caregivers. The needs of this group must be recognized, analyzed and (if they are unmet) satisfied or translated into intervention models. On the one hand, this improves the acceptance of interventions and, on the other hand, increases the probability that a sustainable improvement will occur. I would like to implement this participatory approach with the help of the Alzheimer Research Award2020 of the Hans and Ilse Breuer Foundation. I plan to conduct a comprehensive needs assessment among family physicians in private practice in the care of people with dementia. The general practitioner is usually the first contact person for patients and their relatives, has known these patients for a long time and has often built up a relationship of trust. He is therefore in the first line of care and can implement concepts. For this, however, it is necessary to know what the needs of the doctors are, what they lack in care, where there are obstacles/barriers or also unused opportunities. With the help of the prize money, I would like to collect and analyze this information and ultimately integrate it into the development of further concepts. I hope that research geared to the needs of care will have a lasting effect on the implementation of scientific findings in routine care and can optimize it. This will benefit patients, their relatives and their caregivers in equal measure.
Resume
Jochen René Thyrian was born in Euskirchen on November 12, 1971. He studied psychology at the Julius Maximilians University of Würzburg and graduated in 1999. His diploma thesis was dedicated to the, topic emotionality after a craniocerebral injury. He first worked as a neuropsychologist in the early rehabilitation of neurological patients before moving to the University of Greifswald for his doctorate. In the context of addiction research, he received his doctorate in 2005. Already in addiction research, he conducted intervention studies whose main focus was effectiveness at the population level. Thus, in 2011, he habilitated at the University of Greifswald on the topic of population effectiveness of preventive measures and received the venia legendi in epidemiology and social medicine. Since 2010, he has been conducting research at the German Center for Neurodegenerative Diseases in the field of interventional health services research. Within the framework of a tenure track procedure, a working group was established, which was made permanent after a successful evaluation in 2018. He is a co-opted board member of the German Alzheimer Society and serves on various scientific advisory boards. René Thyrian is happily married and father of two sons. He spends his free time with his family, is passionate about motorcycling and sings in a choir as a tenor.

Director of the Institute for Social Medicine, Occupational Medicine and Public Health (ISAP) of the Medical Faculty of the University of Leipzig
Steffi Riedel-Heller studied human medicine in Leipzig and completed a social psychiatry and psychotherapy specialist training program in Leipzig as well as a Master of Public Health program at Johns Hopkins University in Baltimore, USA. She worked for many years in research, teaching and patient care at the Department of Psychiatry at the University of Leipzig, from 2004 as Professor of Public Mental Health. She wrote her habilitation thesis on the epide-miology of dementia. Since 2010, she has been W3 Professor of Social Medicine and Head of the Institute of Social Medicine, Occupational Medicine and Public Health (ISAP) at Leipzig University. She is a long-standing board member of the German Society for Psychiatry and Psychotherapy, Psychosomatics and Neurology (DGPPN) and the German Society for Social Medicine and Prevention (DGSMP) and on the executive committee of the Association of Scientific Medical Societies (AWMF). She is also managing editor of the Thieme journal “Psychiatrische Praxis”. In 2017, Professor Riedel-Heller received the highly endowed research prize from the Hans and Ilse Breuer Foundation for her work in the field of preventing cognitive disorders in old age. She has published over 850 medline-listed papers to date and is PI of large population-based age cohorts and multicenter intervention studies.
Lecture (PDF document, 3.3 MB) from May 11, 2019 at StattHaus Offenbach of the Hans and Ilse Breuer Foundation.
„Mentally fit into old age – What you can contribute to dementia prevention“.
Research result
Conformation and cell-to-cell transfer of tau molecules in brains with different stages of Alzheimer’s disease-associated neurofibrillary changes.
We are interested in mechanisms of cell-to-cell transmission of tau species and their conformations in brains of different stages of Alzheimer’s disease-associated neurofibrillary changes. Highly sensitive detection methods allow establishing the presence of transneuronal transmission of these tau species even before aggregates of these molecules can be visualized in AT8 immunoreactions. The identification of different tau species in different conformations can provide information about their pathogenicity and their influence on the more or less aggressive progression of the pathological process. Both aspects should make it possible to determine whether cases that are currently widely classified as „primary age-related, non-AD tauopathy“ (PART) can be considered, with good reasons, as separate from the AD-associated process or are in fact on the continuum with it. In addition, we are interested in the postnatal intrinsic development of the pre-α layer (lamina II) of the human entorhinal region and the effects of the AD process on this layer and the tractus perforans. The pre-α layer undergoes the first Alzheimer’s disease-associated changes occurring in the human cerebral cortex. Together with the deep pri-α layer, which is also seized later in the process, these changes disrupt the tight connections between the entorhinal region and the hippocampal formation, as well as those of both areas and the neocortex. Using the CLARITY method, we hope to be able to visualize 3-dimensionally components of the presynaptic and postsynaptic condensations on dendrites of pre-α neurons and thus clearly demonstrate the changes in the connections between neocortex and allocortex (entorhinal region and hippocampus) that occur during the course of the Alzheimer’s associated process.
Interview with Prof. Heiko Braak

Research result
„Drug development for Alzheimer’s disease can be made more efficient and safer“
More than 1 million people in Germany suffer from Alzheimer’s disease. In Alzheimer’s research, we are working at full speed to develop safe and efficient drugs to treat the causes of this disease. Target molecules for drug development include certain enzymes in the brain that act as molecular scissors and cut Alzheimer’s protein into smaller pieces. One of these fragments can form clumps that damage nerve cells in the brain and ultimately lead to Alzheimer’s disease. With the help of the Breuer Prize, we developed new analytical methods, which we then used to find that these molecular scissors not only cut the Alzheimer’s protein, but also other proteins in the brain. However, this means that inhibiting the molecular scissors with drugs could potentially lead to undesirable side effects, as the other proteins would also no longer be cut. To prevent such side effects, we have identified which of the other proteins are particularly important in the brain. Now we are developing diagnostic detection methods to easily measure exactly these proteins and their fragments in the brain fluid and blood. In the future, this will make it possible to find the appropriate dose of the drug for each patient in order to achieve maximum efficiency while minimizing side effects. In summary, the Breuer Prize will help us to make drug development for Alzheimer’s disease more efficient and safer.
Resume
Stefan F. Lichtenthaler studied chemistry at the universities of Karlsruhe, Montpellier (France) and Heidelberg. He then obtained his doctorate at the Center for Molecular Biology at the University of Heidelberg. After a postdoctoral stay at Harvard University (USA), he became a junior research group leader at Ludwig Maximilian University of Munich (LMU), where he habilitated in biochemistry. In 2009, he became department head at the newly founded German Center for Neurodegenerative Diseases (DZNE). Since 2012, he holds the Chair of Neuroproteomics at the Technical University of Munich (TUM) and the DZNE Munich.
Prof. Mikael Simons

Research result
„Deeper understanding of phagocyte function in aging“.
We are interested in the function of phagocytes in the brain. Phagocytes are an important component of the innate immune response and are responsible for the destruction of invading pathogens, but also of endogenous material such as, amyloid plaques. Particle entrapment occurs through the formation of phagosomes, which, after ingestion of the particles, fuse with special vesicles called lysosomes. To study the function of these cells, we inject a toxin into the white matter of the brain to locally damage the myelin sheath. The damaged myelin is then taken up by phagocytes inside the cell and digested. However, if one performs this experiment in older animals one finds an accumulation of undigested residues in the cell. In the older animals, there is deposition of cholesterol in the phagocytes in the lysosomes. The cholesterol originates from the myelin sheaths, which are composed of a high percentage of cholesterol. The accumulation of cholesterol triggers an inflammatory reaction after some time. The deposits occur because phagocytes are unable to break down cholesterol molecules. The excess cholesterol must be removed by lipoproteins. In the brain, this task is primarily performed by apolipoprotein E. These results are relevant for understanding the function of phagocytes in old age.
Resume
Mikael Simons studied medicine in Heidelberg. He received his doctorate in 1998 with a double award-winning dissertation thesis on the molecular mechanisms of Alzheimer’s disease, which was written at the Center for Molecular Biology in Heidelberg. As a postdoctoral fellow and fellow of the German Research Foundation, he worked at the Institute of Neurobiology at the University of Heidelberg. From 2000 to 2004, he was a resident at the Neurological University Hospital in Tübingen. In 2004, he became a specialist in neurology and habilitated at the University of Göttingen in 2005. In 2007, he became head of the Multiple Sclerosis Outpatient Clinic at the Department of Neurology, University of Göttingen. In 2008, he became group leader at the Max Planck Institute for Experimental Medicine and was appointed with a W3 Heisenberg Professorship at the Department of Neurology, University of Göttingen, in 2008.
Prof. Dieter Edbauer

Professor of Translational Neurobiochemistry, DZNE & Ludwig-Maximilians-University Munich
Group Leader of the Helmholtz Junior Research Group
German Center for Neurodegenerative Diseases (DZNE)
Research result
„Toxicity of Dipeptide Repeat (DPR) Proteins.“
An astonishing number of patients suffering from frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) have other affected persons in their family. Genetic studies in these families have identified numerous causative mutations. The most common is a mutation in a poorly characterized gene with the cryptic nameC9orf72, which affects approximately 5-10% of all ALS/FTD patients. Patients with this mutation exhibit a massive elongation of several hundred or even thousand repeats of a (GGGGCC)n sequence in the noncoding portion of theC9orf72 gene. We discovered that the elongated (GGGGCC)n sequence is unexpectedly translated into aggregating proteins in all reading frames. This gives rise to five so-called dipeptide repeat (DPR) proteins (Mori et al, Science 2013), which form numerous deposits in the neurons of patients. The crucial questions now are what role the DPR proteins play in disease development and how toxic effects can be treated with drugs. Thanks to the support of the research award of the Hans and Ilse Breuer Foundation, we were able to investigate the toxic effects of the DPR proteins in cell culture and in mouse models in more detail. We were particularly interested in the association of DPR proteins with the cytoplasmic TDP-43 deposits, because these aggregates are also observed in ALS/FTD patients withoutC9orf72 mutation and are probably a direct cause of cell death. We discovered that DPR proteins interfere with the normal import of TDP-43 into the nucleus, promoting its aggregation in the cytoplasm (Khosravi et al., Hum Mol Genet 2017). Furthermore, we demonstrated an interaction between two of the DPR proteins and ribosomes as well as other RNA-binding proteins. This is thought to chronically disrupt overall cellular translation and contribute to neurodegeneration (Hartmann et al., Life Sci Alliance 2018). In cell culture, we showed that monoclonal antibodies inhibit aggregation and cell-to-cell transfer of DPR proteins (Zhou et al., EMBO Mol Med 2017). Now we are testing the efficacy of this this therapeutic approach in a transgenic mouse model forC9orf72 ALS/FTD that we have previously established and characterized in more detail (Schludi et al., Acta Neuropathol 2017). With part of the prize money, we purchased a 96-channel pipette and other equipment for high-throughput analysis of biological samples. This allowed us to develop a semi-automated method to simultaneously screen hundreds of drugs for efficacy in stem cells fromC9orf72 patients. First, we test a collection of drugs already approved for other diseases. If an already known drug proves to be effective inC9orf72 cells, this would be a promising therapeutic approach with fewer hurdles for a clinical trial in patients in the near future.
Resume
Dieter Edbauer studied medicine in Munich (1994-2000). In his doctoral thesis with Prof. M. Hallek at the Gene Center of the Ludwig-Maximilians-University (LMU) he worked on DNA vaccines against lymphoma (1998-2001). As a physician in internship (AiP), later as a postdoctoral fellow and then as a research assistant, he moved to the Adolf-Butenandt-Institute of the LMU, where he investigated the biochemical mechanisms of Alzheimer’s disease with Prof. C. Haass (2001-2004). As a highlight of the work, a key enzyme of Alzheimer’s disease, the so-called gamma-secretase, could be molecularly defined for the first time. This was followed by a stay abroad at the Massachusetts Institute of Technology in the laboratory of Prof. M. Sheng (2004-2009). The work focused on signal transduction and cell biology in neurons associated with Alzheimer’s disease and fragile X syndrome, a hereditary form of mental retardation. In November 2009, Prof. Edbauer returned to Munich as the first Helmholtz junior research group leader at the newly founded DZNE. Here, new therapeutic approaches are to be identified by analyzing the molecular mechanisms of synaptic dysfunction in Alzheimer’s disease.

Research Findings
„Identification of novel targets for future progression-modified treatment approaches.“
In numerous neurodegenerative diseases, the involvement of inflammatory mechanisms is increasingly emerging as a major component of disease development and progression. Microglial cells as representatives of the innate immune system in the CNS are simulated by aggregates of misfolded proteins or nucleic acids. From their neuroprotective and homeostatic effects under physiological conditions, this activation results in a chronic inflammatory process that contributes to neuronal cell death via pro-inflammatory cytokines. The focus of our work is the NLRP3 inflammasome. This is a signaling pathway that is at the beginning of the activation of the innate immune system and significantly contributes to the development of chronic inflammation in the brain. First, we detected the activation of this inflammatory signaling mechanism in the brains of Alzheimer’s patients using immunohistochemical and biochemical methods. Interestingly, strong activation of the NLRP3 immune mechanism was already detectable in patients with mild cognitive impairment (MCI), suggesting that the observed activation occurs before the dementia stage is reached. In a next step, we demonstrated that genetic blockade of the NLRP3 inflammasome is neuroprotective in a mouse model of AD. Blockade of the NLRP3 inflammasome prevented inflammatory activation of microglia, which subsequently showed enhanced degradation of amyloid deposits in the brains of mice. Of particular importance was the finding that the reduced inflammatory response and enhanced degradation of amyloid deposits resulted in protection of synaptic connections and significantly improved hippocampal function. Since neuroinflammation starts early and before the onset of clinical symptoms of AD, the mechanisms involved are attractive targets for future progression-modifying treatment approaches.
CV
Prof. Heneka is a senior neurologist at the interdisciplinary Clinical Treatment and Research Center (KBFZ) for Neurodegenerative Diseases at the University Hospital Bonn. Michael Heneka graduated in human medicine from the University of Tübingen in 1996. He received his PhD in 1998 at the Institute of Pharmacology and Toxicology on the topic „The effect of polymerized hemoglobin on cardiovascular and renal parameters in septic shock“. He then worked as a postdoctoral fellow in the laboratory of Prof. D.L. Feinstein, University of Illinois at Chicago, Chicago, USA. In 2002 he became a specialist in neurology, and in 2003 he habilitated in neurology at the University of Bonn on the topic of „Inflammatory mechanisms of Alzheimer’s disease: characterization and development of therapeutic strategies“. After a fellowship in the Department of Neurosciences, Case Western Reserve University, Cleveland, USA in the laboratory of Prof. K. Herrup and Prof. G.E. Landreth, he returned to Bonn in 2004 and initially worked as a senior physician in the Department of Neurology. In the same year, he received an appointment to a university professorship (C3) in Molecular Neurology at the Westfälische Wilhelms-Universität (WWU) Münster, where he worked from 2004 to 2008. During this time, he headed the Department of Molecular Neurology and the Dementia Consultation Unit at the University Hospital MS. In 2008, Michael Heneka was appointed as University Professor (W3) of Clinical Neuroscience at the Rheinische Friedrich-Wilhelms-Universität Bonn. Since 2010, Prof. Heneka has been neurological head of the joint Neurological-Psychiatric Memory Outpatient Clinic of the Departments of Psychiatry and Neurology (Clinical Treatment and Research Center, KBFZ), University Hospital Bonn. In addition to his research, review and teaching activities, Prof. Heneka is head of the Clinical Research Group 177 (DFG), board member of the BMBF Competence Network „Degenerative Dementias“ (KNDD) and member of the Bonfor Commission. He is also the organizer of the Venusberg Meeting on Neuroinflammation, which is held every 2 years. In 2011, he received the Christa Lorenz Award for ALS research.
- Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D, Vieira-Saecker A, Schwartz S, Santarelli F, Kummer MP, Griep A, Gelpi E, Beilharz M, Riedel D, Golenbock DT, Geyer M, Walter J, Latz E, Heneka MT. (2017) Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease.Nature 552(7685):355-361.
- Krauthausen, M., Kummer, M.P., Zimmermann, J., Reyes-Irisarri, E., Terwel, D., Bulic, B.,Heneka, M.T.°, and Muller, M.° (2015). CXCR3 promotes plaque formation and behavioral deficits in an Alzheimer’s disease model.J Clin Invest 125, 365-378. °equal contribution.
- Willem, M., Tahirovic, S., Busche, M.A., Ovsepian, S.V., Chafai, M., Kootar, S., Hornburg, D., Evans, L.D., Moore, S., Daria, A., Hampel, H., Muller, V., Giudici, C., Nuscher, B., Wenninger-Weinzierl, A., Kremmer, E.,Heneka, M.T., Thal, D.R., Giedraitis, V., Lannfelt, L., Muller, U., Livesey, F.J., Meissner, F., Herms, J., Konnerth, A., Marie, H., and Haass, C. (2015). eta-Secretase processing of APP inhibits neuronal activity in the hippocampus.Nature 526, 443-447.
- Heneka, M.T., Kummer, M.P., and Latz, E. (2014). Innate immune activation in neurodegenerative disease.Nat Rev Immunol 14, 463-477.
- Heneka, M.T., Kummer, M.P., Stutz, A., Delekate, A., Schwartz, S., Vieira-Saecker, A., Griep, A., Axt, D., Remus, A., Tzeng, T.C., Gelpi, E., Halle, A., Korte, M., Latz, E., and Golenbock, D.T. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice.Nature 493, 674-678.
- Kummer, M.P., Hermes, M., Delekarte, A., Hammerschmidt, T., Kumar, S., Terwel, D., Walter, J., Pape, H.C., Konig, S., Roeber, S., Jessen, F., Klockgether, T., Korte, M., andHeneka, M.T. (2011). Nitration of tyrosine 10 critically enhances amyloid beta aggregation and plaque formation.Neuron 71, 833-844.
- Heneka, M.T., Nadrigny, F., Regen, T., Martinez-Hernandez, A., Dumitrescu-Ozimek, L., Terwel, D., Jardanhazi-Kurutz, D., Walter, J., Kirchhoff, F., Hanisch, U.K., and Kummer, M.P. (2010). Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine.Proc Natl Acad Sci U S A 107, 6058-6063.
- Weberpals, M., Hermes, M., Hermann, S., Kummer, M.P., Terwel, D., Semmler, A., Berger, M., Schafers, M., andHeneka, M.T. (2009). NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits.J Neurosci 29, 14177-14184.
- Heneka, M.T., Ramanathan, M., Jacobs, A.H., Dumitrescu-Ozimek, L., Bilkei-Gorzo, A., Debeir, T., Sastre, M., Galldiks, N., Zimmer, A., Hoehn, M., Heiss, W.D., Klockgether, T., and Staufenbiel, M. (2006). Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice.J Neurosci 26, 1343-1354.
- Sastre, M., Dewachter, I., Rossner, S., Bogdanovic, N., Rosen, E., Borghgraef, P., Evert, B.O., Dumitrescu-Ozimek, L., Thal, D.R., Landreth, G., Walter, J., Klockgether, T., van Leuven, F., andHeneka, M.T. (2006). Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma.Proc Natl Acad Sci U S A 103, 443-448.
- Heneka, M.T., Sastre, M., Dumitrescu-Ozimek, L., Hanke, A., Dewachter, I., Kuiperi, C., O’Banion, K., Klockgether, T., Van Leuven, F., and Landreth, G.E. (2005). Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain 128, 1442-1453.

Short report on the use of funds
Curriculum Vitae

Research result
Curriculum vitae

Research result
Curriculum Vitae

„The physiological function of the Alzheimer’s disease relevant secretases“.
Curriculum vitae

„Membrane trafficking and targeting in Alzheimer’s disease“
Project description
Alzheimer’s disease (AD) is the most common form of neurodegenerative disease and prevalent in the aging population. It is estimated that roughly 37 million people around the world will suffer from some form of dementia by 2025. The most extensive European epidemiological studies (Wimo et al. 2003; Ott et al. 1995) shows that 72% of all dementia patients suffer from AD. An estimated 4.5 million Americans have AD. The number of Americans with AD has more than doubled since 1980 and will continue to grow such that by 2050 the number of individuals with Alzheimer’s could range from 11.3 million to 16 million. There continues to be no cure for AD and basic research continues to fuel efficient drug discovery. By studying the basic biology of Alzheimer’s disease, we aim to develop efficient strategies to inhibit some key processes associated with the disease. A characteristic feature of the disease is the presence of plaques in the brain accompanied by the formation of insoluble tangle-like structures that accumulate inside the brain cells. A predominant hypothesis suggests that a small peptide that usually accumulates in the plaques clumps together and causes the neurodegeneration observed in the disease. This peptide is produced from another molecule, termed amyloid precursor protein (APP), when two enzymes called ß- and ɣ-secretases act on it to release the peptide. By efficiently inhibiting the production of this peptide, one could develop a therapeutic strategy for Alzheimer’s disease. By studying how the cells make this peptide and what are the criteria for the enzymes to produce this peptide, recently our lab developed a new inhibitor for the first enzyme, ß-secretase. In the framework of this project, we aim to study how this peptide becomes toxic in the cells and what are the cellular requirements for this toxicity. If one understands the mechanism by which the cell produces the toxic clumps of this peptide, we could develop novel inhibitors to efficiently inhibit this process for the treatment of Alzheimer’s disease.
CV
| June 2009 – present | University of Zurich, Medical Faculty, Co-Director & Assistant Professor, Systems & Cell Biology of Neurodegeneration |
| Nov 2007 – June 2009 | Max Planck Institute of Molecular Cell Biology and Genetics, Germany, Principal Investigator, Alzheimer Forschung Initiative project & BMBF-FORMAT project “Membrane intervention and systems biology approaches for AD therapy” |
| July 2003 – Nov 2007 | Max Planck Institute of Molecular Cell Biology and Genetics, Germany, Postdoctoral fellow with Kai Simons, M.D., Ph.D. |
| 2003-2007 | Postdoc Cell Biology and Neurosciences, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany (Cell biology of Alzheimer’s disease) |
| 2001-2003 | PhD Immunology (Natural Sciences), University of Konstanz, Konstanz, Germany. Thesis: Role of Membrane microdomains in Leukocyte polarity and signaling |
| 1997-2000 | Pre-PhD Molecular Biophysics, Indian Institute of Science, Bangalore, India Folding of Lectins and artificial chaperone assisted folding of proteins |
| 1995-1997 | MSc Molecular Biology, University of Madras, Madras, India |
| 1992-1995 | BSc Biochemistry, University of Madras, Madras, India |

Research result
„Functional Characterization of Micro-RNA Sequence Variants.“
Alzheimer’s disease (AD) is the most common form of dementia in the population. Certain changes in the DNA sequence of affected patients can either trigger AD (so-called „disease-causing mutations“, very rare) or increase the risk (so-called „genetic risk factors“, common). The biochemical mode of action of genetic risk factors is often much more difficult to determine than that of mutations and is not yet sufficiently elucidated for the vast majority of variants. The overall aim of our project, funded by the Hans and Ilse Breuer Foundation, was to further investigate the possible link between established genetic risk factors of AD and the function of so-called micro-RNAs (miRNAs). MiRNAs are small RNA molecules that bind to so-called messenger RNA (mRNA) molecules and can thus influence the amount of production of proteins (=proteins) encoded by the mRNAs. To investigate this goal, we applied a multifaceted study design combining „in silico“ (i.e., computational) with „in vitro“ (i.e., laboratory) methods. In total, we screened 22 different AD-associated common DNA variants to determine whether they interfere with biochemical binding of miRNA to mRNA. Our in silico predictions here highlighted eight DNA variants, which we subsequently further investigated in the laboratory by applying so-called luciferase reporter experiments and miRNA-specific expression assays to human brain samples. Our results showed that mainly DNA variants in theMS4A6A, FERMT2, andNUP160 genes could affect the binding of miRNA to mRNA, and thus protein synthesis. The results of these experiments were presented in 2013 at the Alzheimer’s Association International Conference (AAIC) in Boston. In another experiment evaluating genome-wide association data of human memory function, we demonstrated that miRNA-138 is a potential regulator of memory function in humans (Schröder et al, 2014).
Since then, our group has intensified its activity in miRNA research and has recently been able to publish several independent papers in this field (e.g. Schulz et al, 2018; Wohlers et al, 2018, Takousis et al, 2019), which would not have been possible without the preliminary work done as part of the Breuer project.
References:
Schröder J, Ansaloni S, Schilling M, Liu T, Radke J, Jaedicke M, Schjeide BM, Mashychev A, Tegeler C, Radbruch H, Papenberg G, Düzel S, Demuth I, Bucholtz N, Lindenberger U, Li SC, Steinhagen-Thiessen E, Lill CM, Bertram L. „MicroRNA-138 is a potential regulator of memory performance in humans.“ Front Hum Neurosci. 2014 Jul 11;8:501. doi: 10.3389/fnhum.2014.00501.
Schulz J, Takousis P, Wohlers I, Itua IOG, Dobricic V, Rücker G, Binder H, Middleton L, Ioannidis JPA, Perneczky R, Bertram L, Lill CM. „Meta-analyses identify differentially expressed micrornas in Parkinson’s disease.“ Ann Neurol. 2019 Jun;85(6):835-851. doi: 10.1002/ana.25490.
Takousis P, Sadlon A, Schulz J, Wohlers I, Dobricic I, Middleton L, Lill CM, Perneczky R, Bertram L. „Differential expression of microRNAs in Alzheimer’s disease brain, blood and cerebrospinal fluid“ Alzheimer’s & Dementia (in press).
Wohlers I, Schulz C, Kilpert F, Bertram L „Alzheimer’s disease risk SNPs show no strong effect on miRNA expression in human lymphoblastoid cell lines“ bioRxiv 367318; doi: https://doi.org/10.1101/367318
What drives you?
What moves you about this topic?
What do you wish for the future?
Curriculum vitae
Research finding
Curriculum Vitae
Research finding
Curriculum Vitae
