Research on dementia in general and Alzheimer’s in particular takes place in many countries and with different goals. We are convinced that dementia is a global problem, which is why a solution can only be found if scientists work together globally. In order to treat an illness effectively, the underlying mechanisms must be understood in detail first.
Basic research is the basis for such knowledge. It takes place in laboratories. Based on their results, applications, innovative technologies and new approaches for the development of medicines and diagnostics arise.
Clinical research, on the other hand, examines the suitability of new diagnostics and the effectiveness of various therapies: drugs or non-drug approaches.
Health services research is interested in the effectiveness of therapies under everyday conditions and also asks how health care can be improved. For people with dementia, health services research investigates how quality of life of affected people can be improved or what support their relatives need.

- The first changes in the brain appear 20 years before the appearance of symptoms.
- Naturally occurring clumps of protein damage the nerve cells in the brain and lead to inflammatory reactions.
- In the end nerve cells in the brain die, causing certain areas in the brain to shrink and to become typically forgetful.
- Currently, only the symptoms but not the causes of Alzheimer’s disease can be treated.
- New drugs that fight the causes are currently being developed.
- The antibodies Lecanemab/Leqembi and Donanemab/Kisunla, can eliminate the protein clumps in the brain and slow down the progression of the disease, but not stop it. This is a breakthrough for Alzheimer’s treatment.
- Leqembi was approved in January 2023 for the treatment of the causes of Alzheimer’s disease in the USA.
- On November 14, 2024, Leqembi was also approved in Europe.
- Donanemab (Kisunla) was approved in the USA and the UK in 2024 and is currently in the approval process in the EU.
- As Leqembi and Kisunla can also cause side effects (occasionally swelling or bleeding in the brain), it will not be suitable for every patient.
- Leqembi, Kisunla and similar drugs are likely to work best if they are used very early after the onset of Alzheimer’s symptoms or even as a preventative measure.
- Other therapeutic approaches are also being developed:
o Stimulation of the immune system to remove protein clumps
o Gamma secretase modulators (GSM) to reduce amyloid beta 42 peptides that tend to aggregate
o Beta-secretase inhibitors for the inhibition of amyloid beta production
o Reduction of the aggregation of the protein tau in nerve cells. - In the future, it may be possible to combine different therapies to treat or prevent Alzheimer’s disease.
Alzheimer’s disease has three major changes in the brain. The brain shrinks because nerve cells die, and there are also two types of protein clumps that are typical of the disease, the so-called amyloid plaques and the bundles of tau fibrils. These changes are caused by several molecular processes in the brain that span a period of 20 to 25 years before the first symptoms of Alzheimer’s disease appear, especially forgetfulness. At the beginning of the molecular processes there is the formation of a protein fragment, which is called amyloid b or Ab and is cut out of an even larger protein by two molecular scissors (so-called secretases). Ab is formed normally in the human body and is not harmful because it is quickly eliminated by the cellular garbage disposal in the brain. As we get older, cellular garbage disposal often doesn’t work as efficiently, so the amount of Ab in the brain increases. As a result, Ab can form clumps, which in turn damage nerve cells and cause the dew protein to form clumps. As the disease develops, both clumps become larger, which leads to the formation of the amyloid plaques and the bundles of tau fibrils. The initially small clumps continue to damage the nerve cells in the brain, leading to inflammatory reactions and ultimately the death of nerve cells in the brain. If a large part of the nerve cells are damaged or dead in certain areas of the brain, symptoms of Alzheimer’s appear. The course of the disease development described here is very well documented by experimental data, including brain imaging, brain fluid examination and genetic mutations that lead to an inherited form of Alzheimer’s disease in a few people or prevent the disease completely.
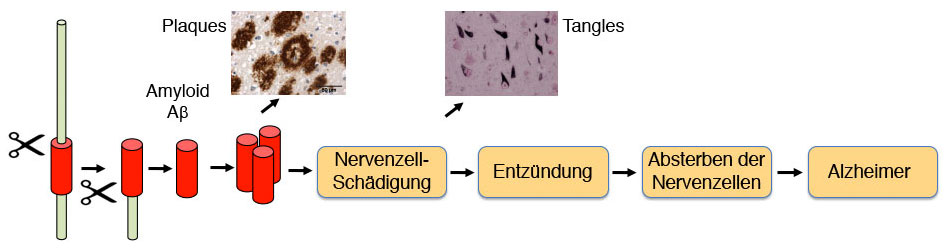
Abbildung 1: Entstehung der Alzheimer Krankheit
When are there drugs to treat the causes of Alzheimer’s disease?
Until now, it has only been possible to treat the symptoms of Alzheimer’s disease, but not the causes. This changed significantly at the beginning of 2023 when immunotherapy with the antibody lecanemab (also known as Leqembi) was approved in the USA. On November 14, 2024, the European Medicines Agency (EMA) also approved it for Europe (https://www.ema.europa.eu/en/news/leqembi-recommended-treatment-early-alzheimers-disease). Other manufacturers are also developing antibodies to remove Aβ aggregates. Donanemab (Kisunla) has already been approved in the USA and the UK and is currently undergoing the approval process in the EU. These immunotherapies are a type of passive vaccination in which antibodies against A are administered by infusion and ensure that A is efficiently removed by the cellular waste disposal system. When given to Alzheimer’s patients, this drug will not stop the disease, but it will slow down its progression, which is a big step forward for those affected because, for example, they can work longer or take care of themselves.
Like many other medications, Leqembi and Kisunla also have side effects and can occasionally lead to swelling or even bleeding in the brain, but this is usually easily treatable and subsides. Nevertheless, the administration of Leqembi must be closely monitored and is not suitable for every patient. For this reason, Leqembi must not be used in Europe for patients with two copies of the ApoE4 gene variant, as they have a high risk of side effects. Since Leqembi and Kisunla intervene in the early steps of the cascade in Figure 1, the antibody therapy will work particularly well in the early phase of the disease, but not once the disease is well advanced. These drugs may even be able to completely prevent the onset of the disease if they are administered preventively. This is being tested in clinical trials.
Another approach involves the direct stimulation of microglia cells. These immune cells in the brain can break down A aggregates. For example, the receptor TREM2, which plays an important role in the degradation of A aggregates, is used to activate microglia cells in a targeted manner.
Given the long phase of disease development (approx. 25 years), it would be particularly useful to stop the development of the disease at an early stage, i.e. before the onset of symptoms, and thus prevent Alzheimer’s disease. Preventing the formation of A or its clumping is an option for prevention. This is being tested with several drugs. One approach is to use drugs to block the upper of the two scissors ( secretase) and thus prevent the formation of A.
Alternatively, the use of gamma secretase modulators (GSM), which reduce the production of amyloid beta 42 peptides that tend to aggregate, is being researched. As these drugs combat the very first cause of Alzheimer’s disease, they are no longer effective once the symptoms of Alzheimer’s disease appear, but must be used specifically for prevention. New studies on prevention are planned. It is a great help that certain brain, cerebrospinal fluid and blood tests can already predict relatively well who is likely to develop Alzheimer’s disease in a few years‘ time and is therefore particularly suitable for preventive treatment. Research is trying to improve early diagnosis even further so that effective preventive treatment can be developed to prevent the disease.
In order to treat Alzheimer’s disease efficiently after it has already broken out, it is important to intervene later in the sequence of the individual disease steps in Figure 1. Drugs are currently being developed that, for example, prevent or remove the tau clumps or suppress the unwanted inflammatory reactions in the brain. However, it will be several years before such drugs are approved.
The brain is a tremendously complex organ, and diseases such as Alzheimer’s and other forms of dementia, in which nerve cells are damaged and destroyed – and so far irreversibly – are correspondingly complex. At the DZNE, the most modern research methods are used to get to the bottom of complex diseases of the brain – such as Alzheimer’s or other forms of dementia. Every month since September 2020, journalist Sabine Heinrich has been asking internationally leading scientists questions about the state of research in the DZNE’s knowledge podcast. The first episode focuses on health services research. The interviewer is Prof. Dr. Wolfgang Hoffmann from the University of Greifswald.
By loading this video, you agree to the privacy policy of Youtube.